|
|
Heterogeneous Catalysis With Nano Clusters
|
|
|
Transition metal and metal compound solids are important industrial heterogeneous catalysts. Neutral metal and metal compound nano clusters composed of limited numbers of atoms in the gas phase, which are fully accessible by both experiment and theory, are excellent model systems through which to investigate the reaction mechanisms for the condensed phase catalytic processes.
|
|
|
Experimental methods
Metal (Mn) and metal oxide (MnOx) clusters are generated by laser ablation with a focused 532 nm Nd:YAG laser onto a metal disk in the presence of pulsed helium carrier gas or an O2/He mixture controlled by a R. M. Jordan supersonic nozzle. Metal and metal oxide clusters are formed in an adjustable length gas channel which is coupled directly to a tube/reactor. The reactant gases are injected into the reactor by a second pulsed valve (General Valve, Series 9). Reactions in the flow tube reactor are estimated to occur at near room temperature due to the large number of collisions between nano clusters and the bath and reactant gases. Ions are deflected from the molecular beam by an electric field and only neutrals enter the ion source of the time of flight mass spectrometer. Ionization is through DUV (193 nm, 6.4 eV), VUV (118 nm, 10.5 eV), and EUV (46.5 nm, 26.5 eV) laser radiation depending on the different clusters and reactants employed. Ions are accelerated and detected by a time of flight mass spectrometer, and signals are recorded and averaged by a digital storage oscilloscope or multi channel scaler.
Computational methods
We employ theoretical quantum mechanical methods (e.g., density functional theory DFT and second order perturbation theory, MP2) to calculate reaction potential energy surfaces, including reaction intermediates, transition states, barriers, and final products to develop a reaction mechanism that explains our experimental data. The experiments inform the theory about the systems to calculate, and the theory explains and motivates the experimental results.
|
|
|
Reaction of Nbn and Tan
clusters with C6H6 and C7H8
|
|
|
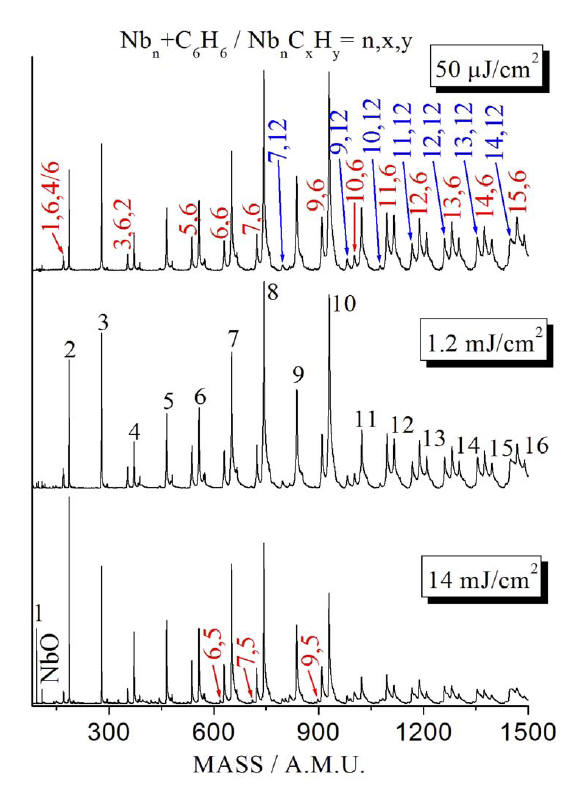
Product
distribution in the reaction of Nbn with ~1 mTorr C6H6
in the pick up cell at three different 193 nm laser fluences. The weak
shoulder on the high mass side of the main peaks is due to oxygen and
carbon impurities
|
|
|
|
|
|
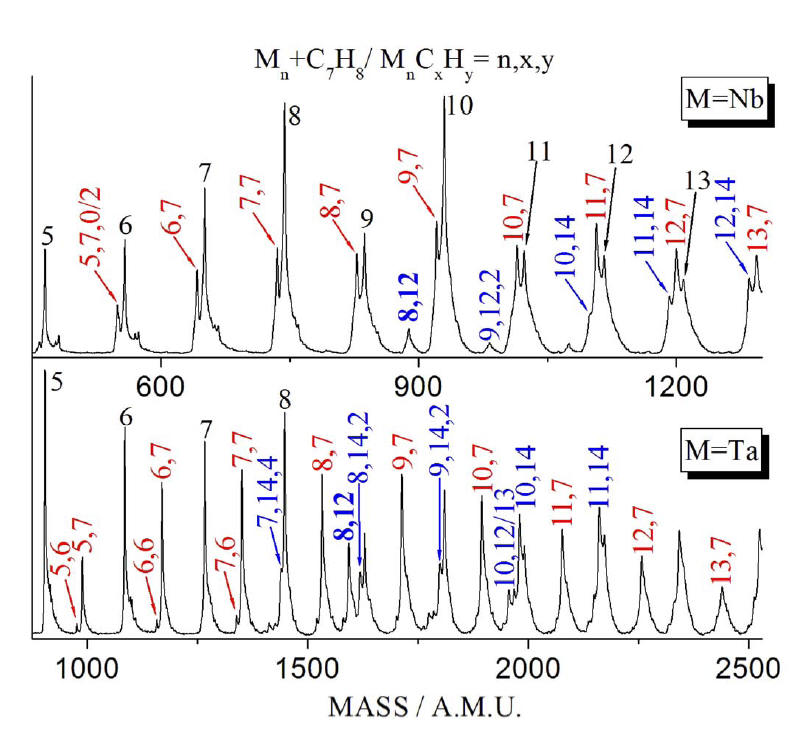
Product distribution in the reaction of
Nbn and Tan with 1.4 mTorr toluene in
the pick up cell. Ionization is by a 193 nm laser with fluence of 770 mJ/cm2
|
|
|
|
|
|
Reaction of Nbn clusters with CO + H2
|
|
|
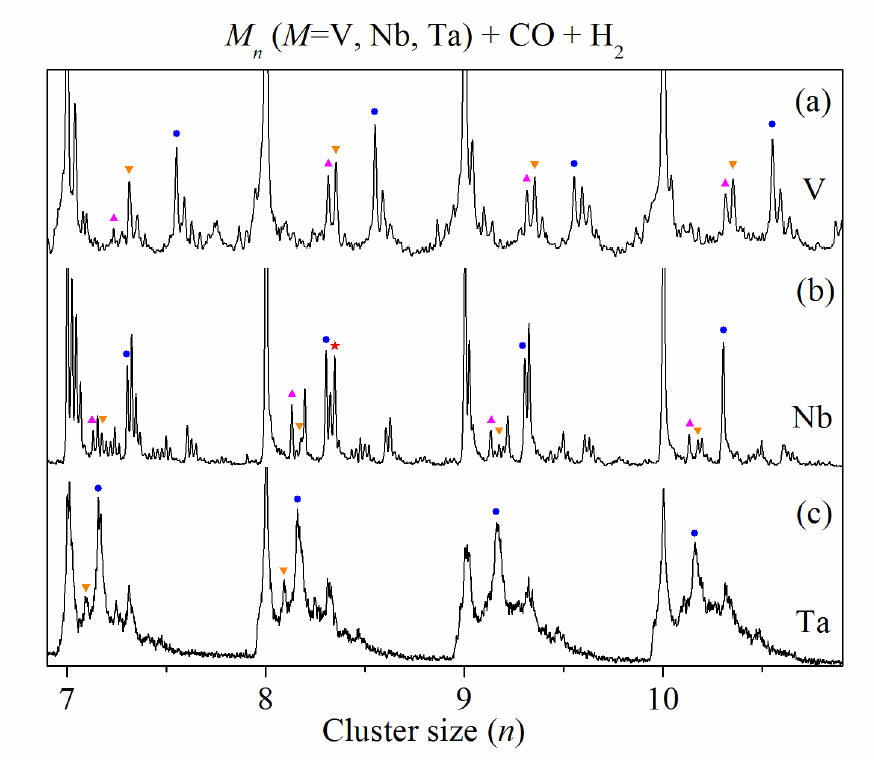
Product
distribution for the reaction of Vn (a), Nbn (b),
and Tan (c) (n = 7-10) with 92 ppm CO + 0.2% H2
in a flow tube reactor. Ionization is by a 193 nm laser at a fluence of 200
mJ/cm2. ▲, ▼, ● and ★ indicate carbide impurities,
oxide impurities, MnCO and Nb8COH4,
respectively. Note the enhanced intensity for the Nb8COH4
feature in the mass spectra
|
|
|
|
|
|
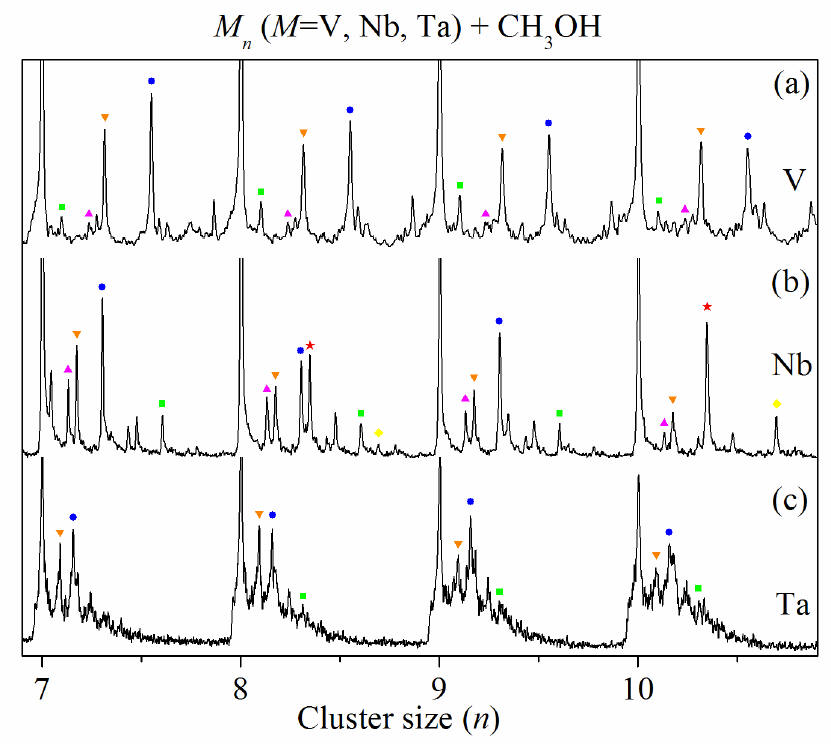
Product distribution for the reaction of
Vn (a), Nbn (b), and Tan
(c) (n = 7-10) with 55 ppm, 92 ppm and 147 ppm CH3OH in a
flow tube reactor, respectively. Ionization is by a 193 nm laser at a
fluence of 200 mJ/cm2. ▲,
▼, ●, ■, ¿ and ★ indicate carbide impurities, oxide impurities, MnCO,
Mn(CO)2, Nbn(COH4)2/Nbn(CH3OH)2
and NbnCOH4/NbnCH3OH,
respectively. Note that except for Nb8 and Nb10, CH3OH
is dehydrogenated on all the metal clusters
|
|
|
|
|
|
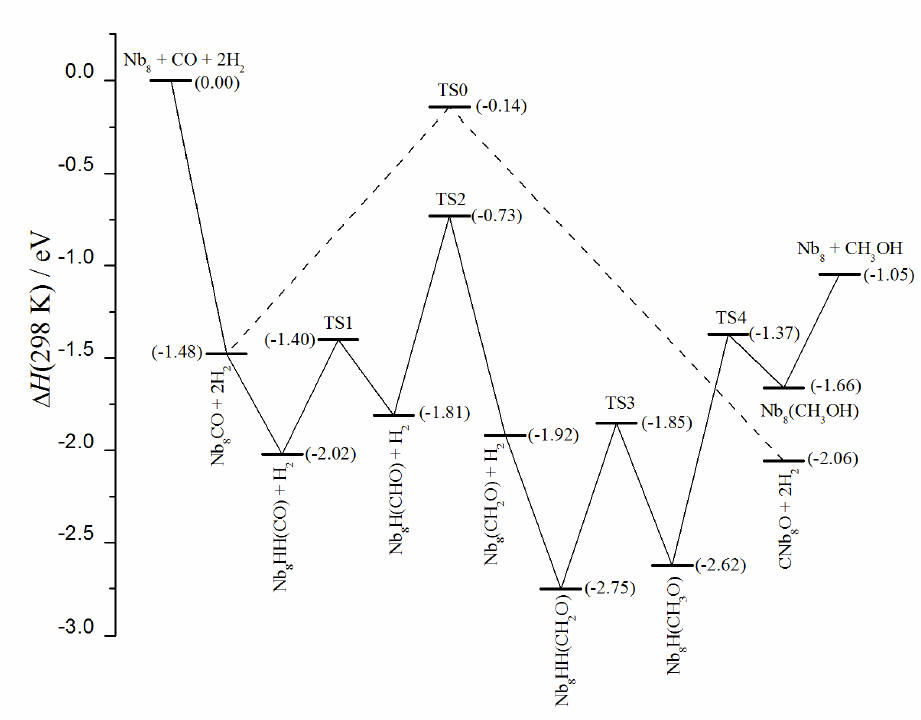
A potential energy surface profile for
the reaction Nb8 + CO + 2H2 → Nb8 +
CH3OH. Energies are in eV and relative to the initial reactant
energy Nb8 + CO + 2H2. Energy levels are calculated
by BPW91/LANL2DZ/6-311+G(2d, p)
|
|
|
|
|
|
Reaction of VmOn clusters with SO2
|
|
|
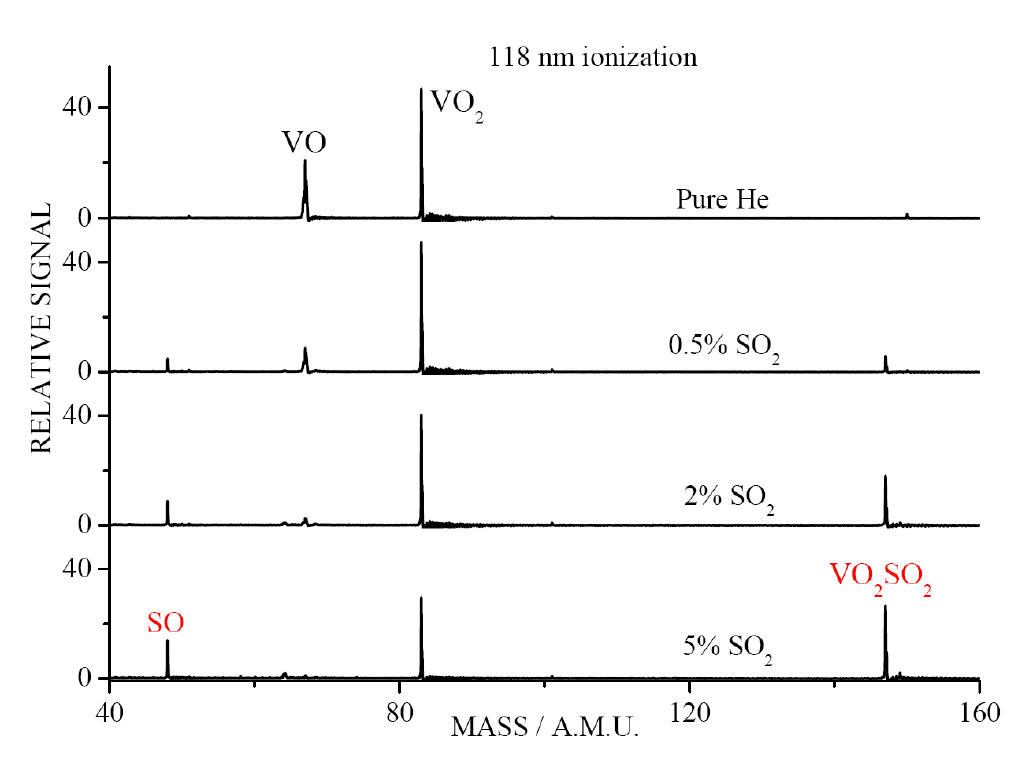
TOF
mass spectra (low mass region) for reactions of VmOn
with different concentrations of SO2 seeded in He. The
concentrations of SO2 are 0%, 0.5%, 2%, and 5% from top to
bottom traces. 1% O2 seeded in He is used to produce VmOn
(this condition is kept to obtain all the TOF spectra reported in the
present work)
|
|
|
|
|
|
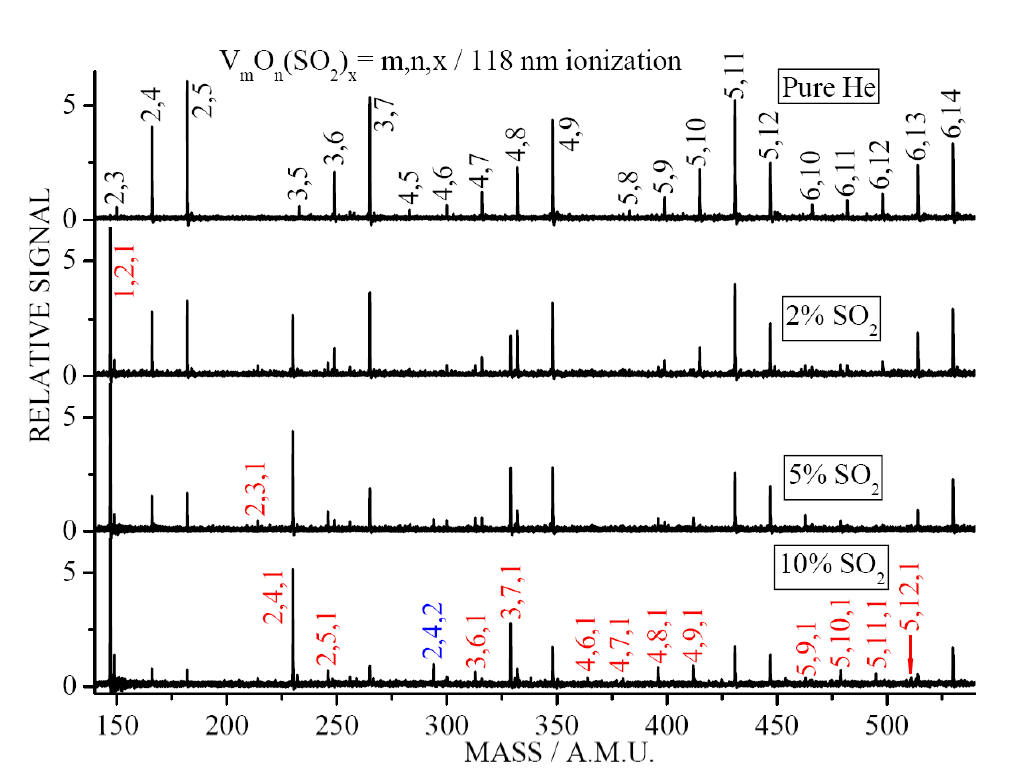
TOF mass spectra for reactions of VmOn
with different concentrations of SO2 seeded in He. The
concentrations of SO2 are 0%, 2%, 5%, and 10% from top to bottom
traces.
|
|
|
|
|
|
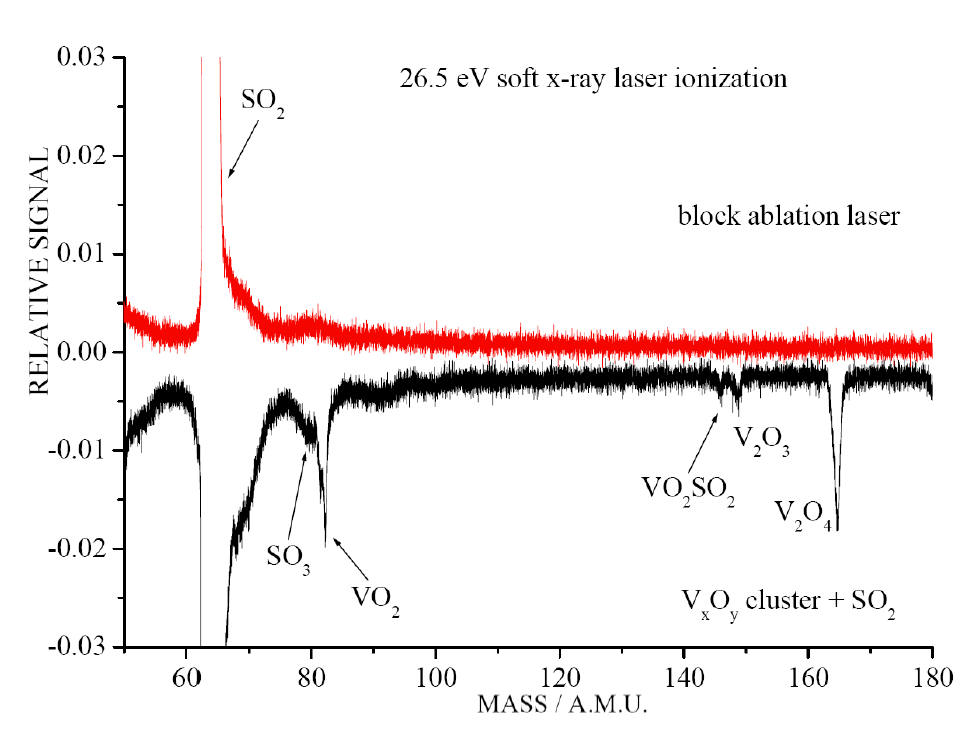
TOF mass spectra for reaction of VmOn
with SO2 employing a 26.5 eV soft x-ray laser for ionization. A
product SO3 is observed when SO2 is added (bottom
trace), and it disappears with the VmOn
cluster signal when the ablation laser is blocked (top trace)
|
|
|
|
|
|
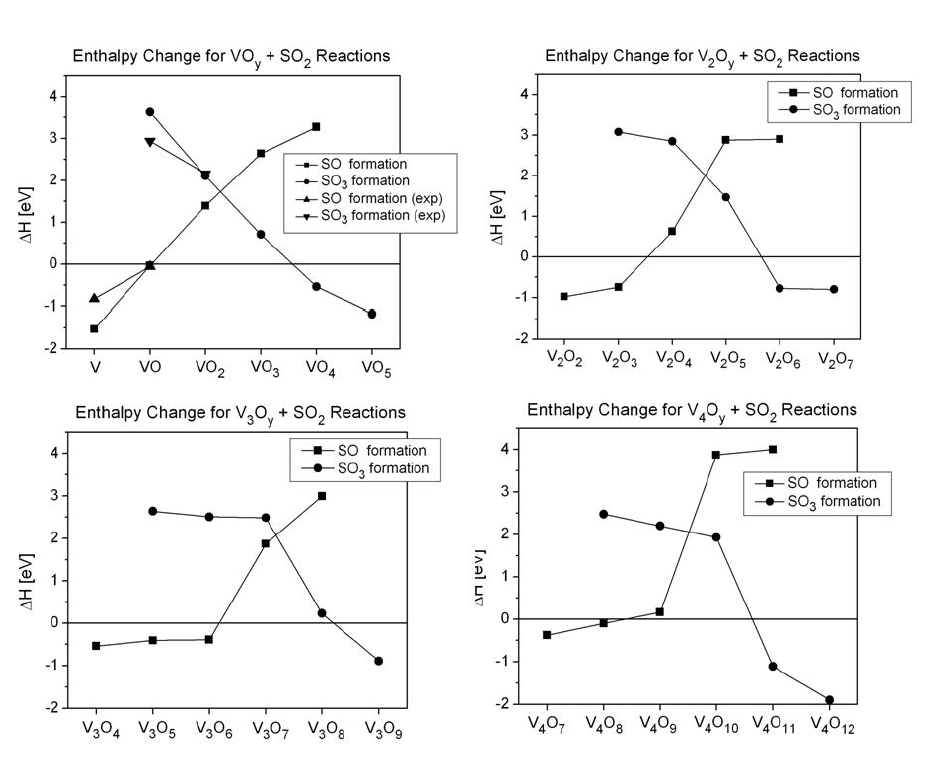
Enthalpy (at 298.15 K) for VxOy
+ SO2 -> VxOy+1
+ SO and VxOy + SO2 -> VxOy-1
+ SO3 reactions.
|
|
|
|
|
|
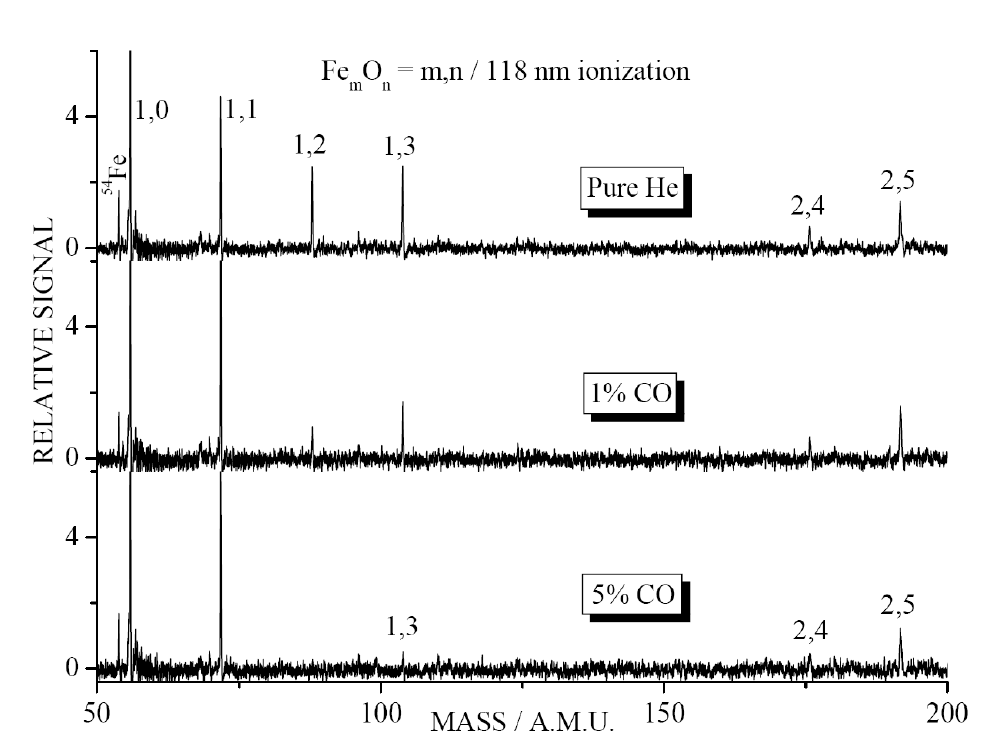
TOF mass spectra for reaction of neutral
iron oxide clusters with carbon monoxide in flow tube reactor. CO
concentrations are 0% (top trace), 1% (middle), and 5% (bottom) in helium
carrier gas
|
|
|
|
|
|
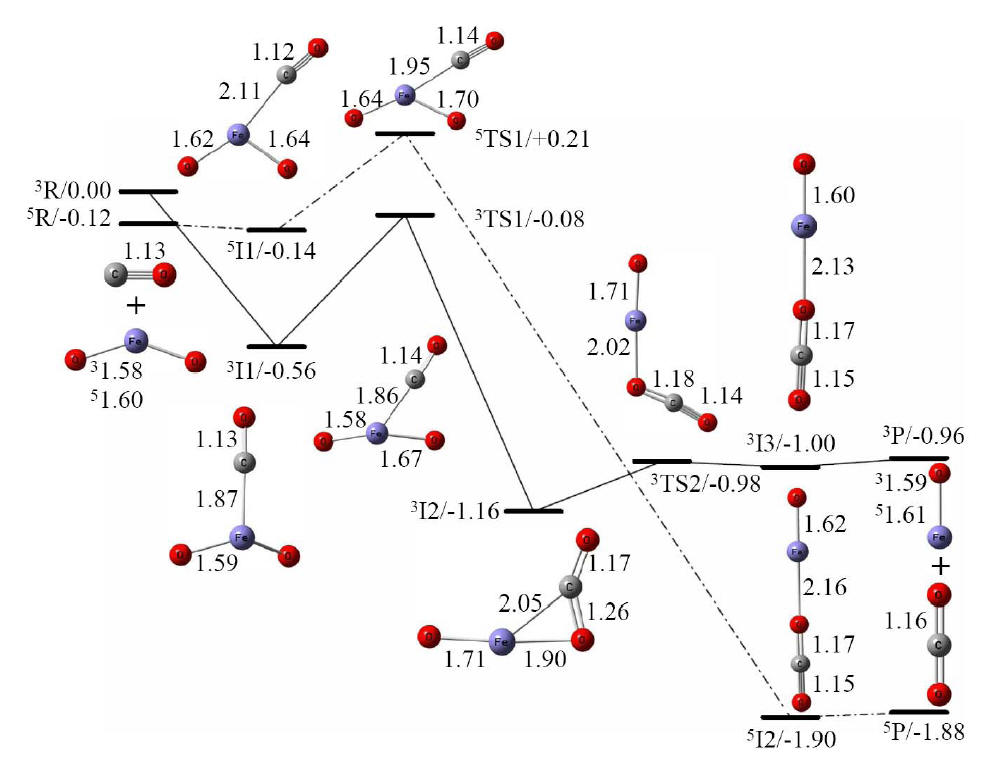
DFT calculated reaction pathways of FeO2
+ CO → FeO + CO2. Reaction intermediates (In),
transition states (TSn), and their relative Gibbs free energies
(ΔG in eV) at 298 K are given as In/ΔG or TSn/ΔG.
Bond lengths in Å are also given for each geometry. Superscripts 3 and 5
are used in this figure to differentiate the reactants, products,
intermediates, and transition states in triplet and quintet spin states,
respectively
|
|
|
|
|
|
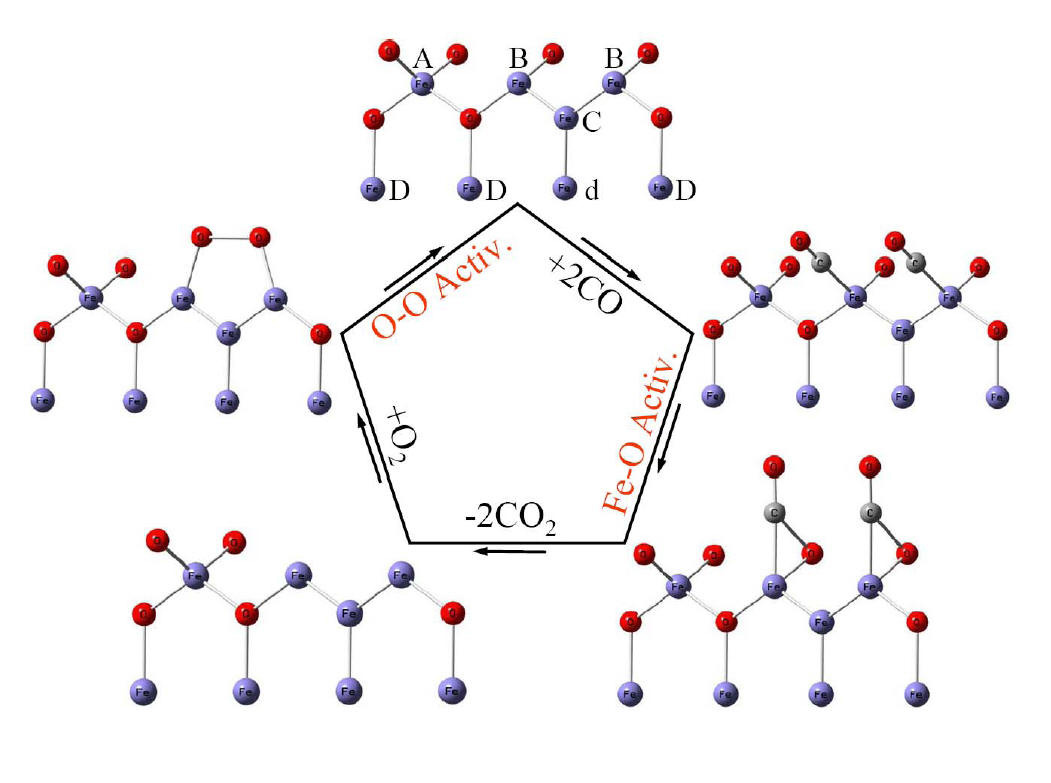
A possible catalytic cycle for CO
oxidation by O2 over iron oxide catalysts at the molecular
level. A, B, and C mark different surface Fe atoms while D and d mark
lattice Fe atoms that are underneath the surface.
|
|
|
|